Tactical medics worldwide use the Abdominal Aortic and Junctional Tourniquet–Stabilized (AAJT-S) to control hemorrhage from armpit, groin and pelvic injuries.
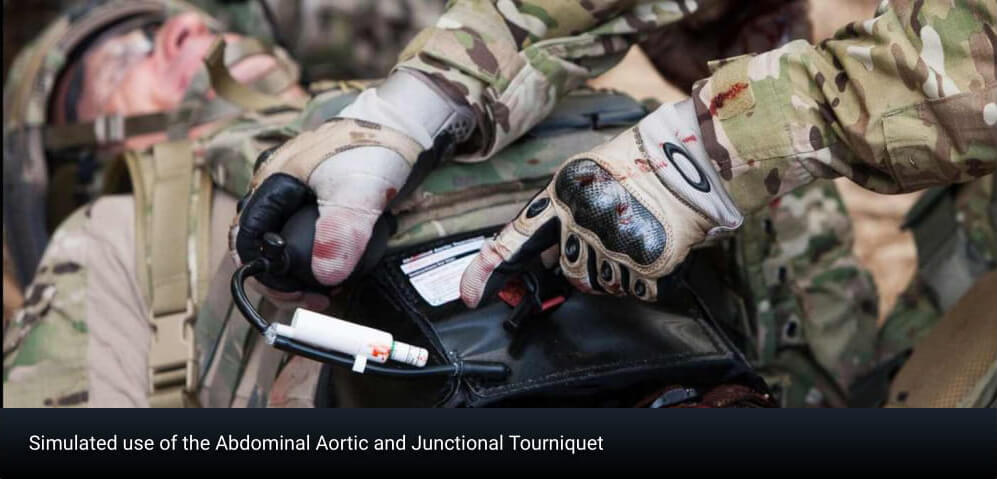 Tactical medics worldwide use the Abdominal Aortic and Junctional Tourniquet–Stabilized (AAJT-S) to control hemorrhage from armpit, groin and pelvic injuries. Research over the last Zve years has proven that the AAJT-S can effectively halt massive bleeding, and battleZeld experience shows that the device can save lives. But what about complications?
Tactical medics worldwide use the Abdominal Aortic and Junctional Tourniquet–Stabilized (AAJT-S) to control hemorrhage from armpit, groin and pelvic injuries. Research over the last Zve years has proven that the AAJT-S can effectively halt massive bleeding, and battleZeld experience shows that the device can save lives. But what about complications?
When applied to the abdomen, the AAJT-S compresses the descending aorta to occlude blood flow to the pelvis and extremities. Some medical professionals have
expressed concern that extended compression of the abdomen can lead to bowel ischemia and other problems. What do animal and human studies indicate?
According to Richard Schwartz, MD, co-inventor of the AAJT-S, research consistently shows that abdominal application is safe when used within the FDA-cleared application time of 60 minutes. In addition, at the inguinal or axillary application sites, the AAJT-S has the same 4-hour recommended application time as other junctional tourniquets.
“While recent papers identify potential complications after extended application of the device, these studies actually reinforce the Znding that the AAJT-S is safe when used as indicated,” Dr. Schwartz said.
“Beyond that, the research also sheds light on how to think about the AAJT-S when making real-life ‘risk This article was developed by Trauma System News in cooperation with our advertiser,
Compression Works.“ AAJT-S abdominal placement (click to enlarge) versus beneZt’ decisions.”
Swedish military study
Recently, researchers funded by Swedish Defense examined the effects of prolonged abdominal
placement of the AAJT-S. Their Zndings were published online in the Journal of Trauma and Acute Care Surgery in July 2018.
In the study, 15 pigs were subjected to a class II hemorrhage. Five received the AAJT-S for 60 minutes and Zve received the device for 240 minutes (there was also a ]uid-only control group). Here are the key results:
The AAJT-S was hemodynamically and respiratorily tolerable for all animals in the study.
Animals in the 240-minute group showed signs of small intestine and liver ischemia, hyperkalemia, metabolic acidosis and other complications.
Animals in the 60-minute group displayed only minimal complications, and all consequences of reperfusion were reversible.
“In this study, there were really no substantial complications associated with abdominal application of the AAJT-S up to one hour,” Dr. Schwartz said. “After four hours of application, predictably, there were complications. However, with any tourniquet, if you leave it on
long enough you will develop ischemia.”
These Zndings align with early tests of the AAJT-S. “In our preliminary data, we began to see a rise in potassium after about 90 minutes of application,” Dr. Schwartz said. “That’s why when we submitted our premarket notification to the FDA, we specified a 60-minute application time when used on the abdomen for aortic occlusion.”
Although extended application led to signiZcant complications in this study, Dr. Schwartz noted that the survival rate was 100% in an intensive care setting. “They were actually able to resuscitate all the animals, even after 240 minutes of application.”
U.S. Army study
In a similar investigation, researchers from the U.S. Army Institute of Surgical Research studied
abdominal application of the AAJT-S for 60, 90 and 120 minutes. Their results were published online in the Journal of Surgical Research in June 2018. Here are the main Zndings:
All animals in the 1-hour group recovered full mobility within 1 week. Damage to muscle, nerve and intestinal tissue was insignificant or undetectable.
Half of the animals in the 1.5-hour group developed persistent hind leg paraplegia.
All animals in the 2-hour group developed persistent paraplegia and other complications.
“These results suggest that the timeframe where problems start to develop is between 1.5 and 2 hours,” Dr. Schwartz said. “But this paper essentially shows the same Znding as the Swedish study — at 60 minutes of abdominal application the AAJT-S is safe.”
Dr. Schwartz believes the complications data should be viewed in relation to other interventions for massive bleeding. For example, a recent military study demonstrated that in terms of physiology, abdominal application of the AAJT-S is essentially equivalent to Zone 3 REBOA. “The complications associated with the AAJT-S after extended application are very similar to what we see with REBOA within these timeframes,” he said.
He also believes the data sheds light on the question of risks versus beneZts. “The AAJT-S is indicated for individuals with devastating injuries in situations where there really is no other means of controlling the hemorrhage,” Dr. Schwartz said. “In these situations, the choice may be between a dead casualty and a casualty with ischemic gut if application goes over 60 minutes.”
Future directions
“Every study shows that abdominal application of the AAJT-S is safe for 1 hour — in my opinion that question is answered,” Dr. Schwartz said. New research will examine just how far the window of safety extends. “We need to do more work to identify the ‘in]ection point’ where you begin to see irreversible ischemic change.”
Dr. Schwartz is Chairman of Emergency Medicine and Hospitalist Services at the Medical College of Georgia at Augusta University. He and his colleagues are now designing a study that will look at AAJT-S complications that emerge between 1 and 2 hours of abdominal placement.
Future studies may also investigate the AAJT-S as an intervention for unstable pelvis. “The AAJT-S is the only FDA-cleared solution for the prevention of shock in pelvic bleeding,” said John Croushorn, MD, co-inventor of the device. “So pelvic fracture is an area we have a lot of interest in.”
Other investigations could focus on the AAJT-S as an adjunct to resuscitation. A recent U.S. Air Force study suggests that abdominal application of the AAJT-S could dramatically increase survival for victims of traumatic cardiac arrest.
“The AAJT-S has the ability to improve cerebral perfusion during CPR, so we think it holds substantial potential for use in cardiac arrest and other resuscitation scenarios,” Dr. Schwartz said. “Overall, we think we are just beginning to scrape the surface on the potential applications of this device.”


