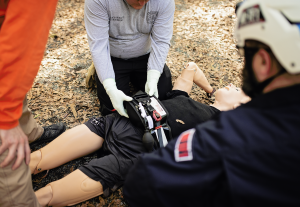
Zaporizhzhia, Ukraine – September 30, 2025 – In a remarkable case that underscores both clinical ingenuity and the global urgency of maternal health, Ukrainian physicians turned to a battlefield bleeding control intervention in a last-ditch effort to save a young mother experiencing severe postpartum hemorrhage (PPH). The case marks the first documented use of the Abdominal Aortic and Junctional Tourniquet – Stabilized (AAJT-S) to manage uncontrolled bleeding after childbirth — with life-saving results.
Olya, a mother of five, had just completed what appeared to be a fast, routine labor when she suddenly began to bleed uncontrollably. Standard interventions, including uterotonics such as Pitocin, failed to stop the hemorrhage. Facing a rapidly deteriorating situation, Dr. Yevheniia Poliakova made the decision to use the AAJT-S.
Dr. Yevheniia Poliakova, the attending physician, had previously studied advanced hemorrhage control tools used in military medicine, including the Abdominal Aortic and Junctional Tourniquet – Stabilized (AAJT-S) — an FDA-cleared device indicated to control difficult-to-manage bleeding in the abdomen, pelvis, inguinal region, and axilla.
“She was one of four patients in our feasibility study using the AAJT to treat postpartum hemorrhage, and her bleeding completely stopped following the application of the device,” said Dr. Yevheniia Poliakova. “It gave us the time we needed to stabilize her and begin surgical intervention. We have since gone on to use the AAJT in four additional patients, bringing our total to eight — with 100% success and no complications.”
Olya remembers only fragments of the day — but enough to understand just how close she came to losing her life.
“This was my fifth delivery. I have five wonderful children, all delivered by Dr. Yevheniia,” Olya said. “Everything went very well, but very quickly — just around two hours. When the baby was already in my arms, I felt okay. But then, something started to feel wrong. Doctors kept checking on me. Then I started to feel… not very good. They gave me medicine and said, ‘Don’t worry, you will sleep now. Everything will be fine."
“The next thing I remember, I woke up in the hospital. I was alive. My baby was there. I don’t know how the device works. But I believe, thanks to it — and thanks to my doctor — I’m here. I’m alive for my children.”
A Global First in Postpartum Hemorrhage Care
The initiative was led by Dr. Viktor Oshovskyy, whose foresight enabled exploration of trauma technology in obstetric emergencies. To date, eight patients with severe postpartum hemorrhage have been treated using the AAJT-S at the facility — with 100% survival and no complications reported.
This case marks the first documented use of the AAJT-S in a postpartum hemorrhage setting — and adds to the more than 70 peer-reviewed studies and growing clinical evidence supporting its use in managing life-threatening hemorrhage.
Originally developed for battlefield trauma, the AAJT-S is the only device FDA-cleared to occlude blood flow in the abdominal aorta — enabling rapid hemorrhage control when standard interventions fail. While not yet indicated for obstetric use, this case study offers a compelling look at how trauma-informed technologies may help address one of the leading causes of maternal death worldwide.
“Dr. Poliakova acted with courage and clarity in an environment where maternal hemorrhage claims lives far too often,” said Scott Dodson, CEO of Compression Works. “What she did — and what her patient survived — matters deeply in this moment of global maternal health crisis.”
A Call for Global Attention
Globally, postpartum hemorrhage affects more than 14 million women each year and is responsible for approximately 70,000 maternal deaths — accounting for over 20% of all maternal mortality worldwide. Maternal mortality from hemorrhage remains a devastating challenge — particularly in resource-limited settings across Eastern Europe, Sub-Saharan Africa and Southeast Asia. From Ukraine to Uganda, emergency obstetric care teams continue to seek better tools, faster responses, and more adaptable treatment options.
This case study points to the urgent need for adaptive technologies that can be deployed rapidly in both hospital and prehospital environments.
About the AAJT-S
The Abdominal Aortic and Junctional Tourniquet – Stabilized (AAJT-S) is an FDA-cleared and CE-marked hemorrhage control device designed to occlude blood flow in the abdomen, pelvis, inguinal region, and axilla. It is the only device of its kind capable of achieving aortic occlusion outside the operating room. The AAJT-S is currently used across military, prehospital, and hospital settings around the world.
See the published abstract: Temporary aortic occlusion with the abdominal tourniquet for refractory postpartum hemorrhage available via PubMed
HEMORRHAGE STOPS HERE!
About Compression Works
The AAJT-S is designed by a team dedicated to addressing the most pressing needs of emergency and battlefield medicine.
For more information about Compression Works, the AAJT-S, and our latest news and studies, visit our Newsroom.
Scott Dodson, CEO
1 800-988-4052
John Croushorn MD, FACEP, CMO
1-800-988-4052
www.compressionworks.com
Visit us on social media: